Sickle Cell Patient Describes ‘Rebirth’ After Gene Therapy
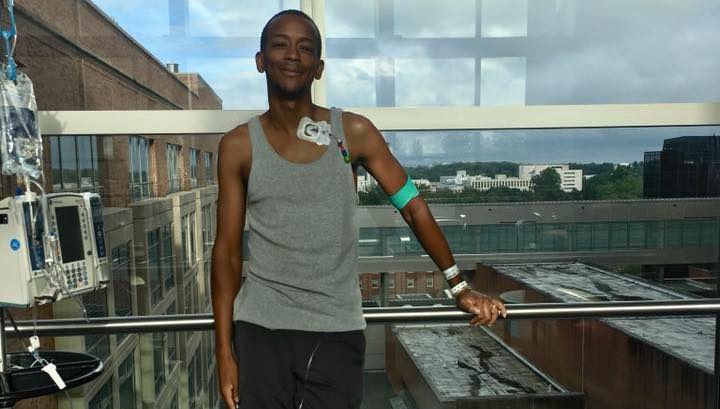
Charles Hough poses for a picture only a few days after his stem cell therapy. (Photos courtesy of Charles Hough)
For most of his 39 years, Charles Hough lived with extreme pain caused by sickle cell disease, which dramatically reduced blood flow throughout his body. But thanks to the gene therapy he received in 2017, Hough is now symptom-free.
“I feel like I have a new chance at life, a healthy, full life without any complications,” Hough said in a recent webinar organized by the National Organization for Rare Disorders (NORD) and the American Society for Gene and Cell Therapy.
Hough said his long journey to a cure began while shopping at Walmart, when he overheard the parent of a boy with sickle cell disease talking about how well he was doing in a clinical trial underway at the National Institutes of Health (NIH) in Bethesda, Maryland. Hough did his own research and ultimately decided to participate in the same trial, signing the necessary consent forms in March 2017.
The first step in the lengthy process was a series of blood transfusions lasting a year and a half, which kept his hemoglobin level high and his sickle blood cell count low.
Researchers then collected hematopoietic stem cells through four bone marrow aspirations. They sent the liquid samples of Hough’s bone marrow to a lab, where his mutated HBB gene was replaced with a healthy copy. Doctors also delivered a healthy copy of the gene to the rest of Hough’s cells through a viral-based carrier. During the infusion of healthy genes, Hough said he experienced a heart attack and silent stroke.
Finally, on Sept. 25, 2018 — the day NIH personnel refer to as Hough’s “rebirth” day — researchers transplanted Hough’s edited stem cells back into his body.
Hough underwent one session of chemotherapy to ensure that his body would not reject the genetically edited stem cells. But the chemo took a heavy mental and physical toll; he lost his hair and became gaunt.
“I looked in the mirror and didn’t notice myself,” Hough said. “That hurt and I broke down.”
The NIH kept Hough for an extra 27 days so he could fight any infections as a result of his weakened immune system. During that time, Hough said he was in and out of consciousness and doesn’t remember a lot.
“Now that chemo had done its part all I had to do was get better,” Hough said. “I was just so tired, I slept.”
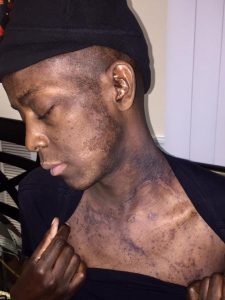
Even outside of the hospital he experienced gastrointestinal problems and hyperpigmentation that lasted three to four months after his “rebirth” day.
In June 2018, Hough did something he never could have before: he traveled to the Philippines, where he drove a scooter for two hours, jumped into cold water and explored caves. Through the 15-day trip, he had no complications.
Before, Hough would worry about being sick or experiencing chronic pain wherever he went. The disease also would limit his ability to work, forcing him to call in sick several times a week. Thirty-seven percent of his blood is now made up of sickled red blood cells instead of 97%.
“It’s the flip side of the coin every day. Every morning I feel healthy,” Hough said. “There’s no more looking back.”
The webinar was the last in a five-part series on gene therapy and was sponsored by Amicus Therapeutics, Avrobio, Bluebird bio and Sarepta Therapeutics.
The NORD webinar also included testimony from Nicole Almeida, whose newborn son received gene therapy after being diagnosed in utero (before birth) with spinal muscular atrophy (SMA).
NORD’s next webinar, set for Jan. 23, will be on patient engagement and the U.S. Food and Drug Administration (FDA). Titled “Your Voice Matters,” it will feature four top FDA officials: Andrea Furia-Helms, director of patient affairs staff at the Office of Clinical Policy and Programs; Michelle Tarver, patient science and engagement program director at the Center for Devices and Radiological Health (CDRH); Robyn Bent, director of the patient-focused drug program at the Center for Drug Evaluation and Research (CDER); and Diane Maloney, associate director for policy at the Center for Biologics Evaluation and Research (CBER).
Information about registering for the event is available here.