Abnormal Flow of Sickled Cells Damages Blood Vessel Walls
Written by |
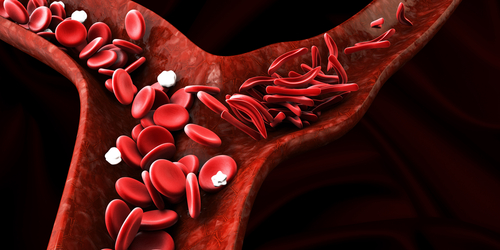
Red blood cells in people with sickle cell disease (SCD) flow through blood vessels in a manner that damages the walls, which may explain blood vessel problems and increased inflammation in SCD, new research indicates.
The study, “Flow-induced segregation and dynamics of red blood cells in sickle cell disease,” was published in Physical Review Fluids.
SCD is caused by an abnormal version of hemoglobin, a molecule used by red blood cells to ferry oxygen through the bloodstream. A consequence of this abnormal hemoglobin is that red blood cells, which are disk-shaped in healthy people, instead acquire a “sickle-like” shape, from which the disease gets its name.
Other characteristic biological features in SCD include the abnormal function of the endothelium (the lining inside blood vessels) and increased inflammation. However, the mechanisms that drive these phenomena are poorly understood.
In the new study, researchers in the U.S. used computer simulations to model how healthy and sickled red blood cells flow through blood vessels.
The main finding of their simulation was that normal, disk-shaped cells tend to congregate in the center of blood vessels as they flow through, leaving a “cell-free layer” next to the endothelium. In contrast, sickled cells often flow in the layer that is normally cell-free, causing them to bump up against the endothelium.
“Our new simulations showed how the motions of sickle cells near vessel walls generate large forces [on the endothelium],” study co-author Michael Graham, PhD, said in a press release. Graham is a professor of chemical and biological engineering at the University of Wisconsin-Madison.
It is known that physical stress on endothelial cells — of the sort that would be generated by sickled red blood cells bumping against them – can induce biological processes like inflammation and endothelial defects. As such, “these results may aid in understanding mechanisms for endothelial dysfunction associated with SCD,” the researchers concluded.
The researchers’ simulations were done using the Comet system at the San Diego Supercomputer Center (SDSC) on the University of California San Diego campus.
“Large-scale shared computing resources such as Comet open doors to doing simulations that could not be done with the resources of an individual research group,” Graham said.
Co-author Wilbur Lam, MD, a physician and biomedical engineer at Emory University and the Georgia Institute of Technology, added: “We are grateful for being able to use supercomputers such as Comet to help us better illustrate, and hopefully come closer to a cure for, sickle cell disease.”





