Casgevy (exagamglogene autotemcel) for sickle cell disease
What is Casgevy for sickle cell disease?
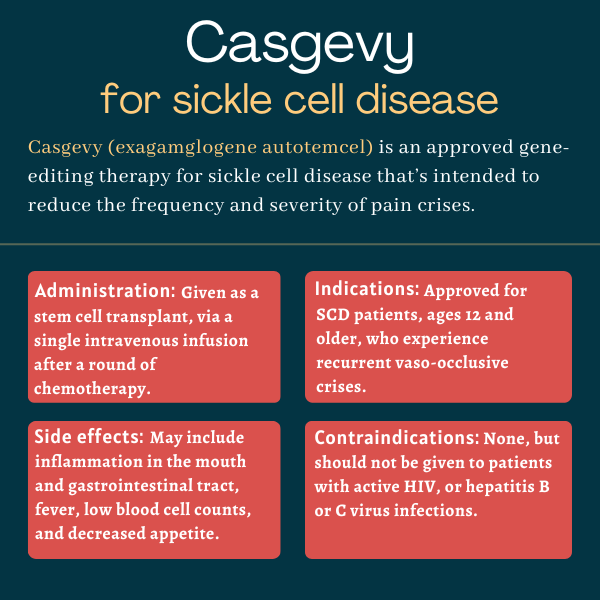
Casgevy (exagamglogene autotemcel), formerly known as CTX001 or exa-cel, is an approved gene-editing therapy used for people with sickle cell disease (SCD) who experience recurrent vaso-occlusive crises (VOCs). It is designed to reduce the frequency and severity of these painful crises.
The therapy, administered via a one-time intravenous or into-the-vein infusion, was developed by CRISPR Therapeutics and Vertex Pharmaceuticals.
In addition to SCD, Casgevy also is approved to treat a related condition called transfusion-dependent beta thalassemia (TDT).
Therapy snapshot
| Brand name: | Casgevy |
| Chemical name: | Exagamglogene autotemcel |
| Usage: | Used to reduce the frequency and severity of vaso-occlusive crises in sickle cell disease |
| Administration: | Intravenous infusion |
How does Casgevy work?
Sickle cell disease is caused by mutations in the HBB gene that result in the production of a faulty version of hemoglobin — the protein in red blood cells that’s responsible for oxygen transport.
The defective version of the protein tends to form clumps inside red blood cells, changing their shape to one that is sickle-like. These sickled red blood cells are more prone to die prematurely and obstruct blood vessels, leading to anemia, vaso-occlusive crises or VOCs, and other sickle cell symptoms and complications.
There are several versions of hemoglobin in the body. One of them, called fetal hemoglobin, is produced during fetal development and is most effective at carrying oxygen. Its production is switched off sometime after birth and replaced by its adult counterpart, which is the version affected by SCD-causing mutations. That switch is mediated by a protein called BCL11A, which basically instructs the body to slow fetal hemoglobin production and make more of the adult type.
Casgevy uses a gene-editing technology called CRISPR/Cas9 to boost the production of fetal hemoglobin by modifying the gene that encodes BCL11A and reducing its activity. This lowers the production of BCL11A, enabling more fetal hemoglobin to be generated and preventing red blood cell sickling.
The treatment involves collecting a patient’s hematopoietic stem cells — the stem cell precursors that give rise to all mature blood cell types — and editing them in a lab. The cells are then returned to the patient via a stem cell transplant.
The edited stem cells are then expected to engraft, or survive and multiply, in the bone marrow, giving rise to mature red blood cells capable of producing healthy fetal hemoglobin. Prior to the infusion, patients must also undergo a round of high-dose chemotherapy to kill off unhealthy cells and make room for the edited new ones.
Who with sickle cell disease can take Casgevy?
Casgevy was approved by the U.S. Food and Drug Administration (FDA) in December 2023 for the treatment of SCD patients, ages 12 and older, who are experiencing recurrent VOCs. With that decision, Casgevy became the first ever approved therapy to employ CRISPR gene-editing technology in the country.
The therapy also has been conditionally approved in the U.K. since November 2023. In that country, it may be used for SCD patients ages 12 and older for whom a stem cell transplant is appropriate, but no donor is available, and who experience recurrent VOCs.
In the U.K., Casgevy also earned approval for the treatment of TDT. The FDA also approved Casgevy for the treatment of TDT in patients ages 12 and older in January 2024.
Casgevy also earned conditional approval in the European Union to treat SCD and TDT in patients ages 12 and older, similar to the U.K.
Who should not take Casgevy?
No contraindications are listed on Casgevy’s prescribing information. However, the medication should not be used in people with an active human immunodeficiency virus or HIV infection. It also should not be given to patients with hepatitis B virus or hepatitis C virus infections.
How is Casgevy administered in sickle cell disease?
There are several steps involved in the process of manufacturing each patient’s specific Casgevy formulation, preparing the body for treatment, and administering the therapy. The length of time the entire process takes will vary from patient to patient, but may take up to a year.
Deciding if Casgevy is appropriate, and then starting the treatment process if so, can be done only at an authorized treatment center. There, it will be determined if a patient is eligible for a stem cell transplant. Patients also will be screened for infectious agents, including HIV and hepatitis B and C, prior to initiating the process.
Once Casgevy is deemed appropriate, other disease-modifying SCD therapies may need to be stopped — typically eight weeks prior to starting the treatment process — which will be determined at the discretion of a healthcare provider. Patients also may receive extra blood transfusions at this time to help maintain adequate hemoglobin levels.
Stem cell collection
First, patients will have to be treated with a medication like plerixafor, which causes their hematopoietic stem cells to move out of the bone marrow and into the bloodstream, inducing a process known as stem cell mobilization. Cells then will be collected via a process called apheresis, and used to manufacture Casgevy.
This cell collection process will be repeated on two, and possibly three consecutive days. If not enough cells are collected, patients may need to undergo an additional round of cell collection. That would occur at least two weeks later, after the body has had time to make more stem cells.
The collected cells then are sent to a lab, where they are edited, frozen as a cellular suspension, and sent back to the patient’s healthcare provider. This process may take up to six months. Some collected cells also will remain unedited and stored in case there is a need for a rescue stem cell transplant if Casgevy can’t be administered or does not work.
Conditioning regimen
Two to seven days before the Casgevy infusion — after the medication has been manufactured and stored at the patient’s treatment center — the patient will undergo a myeloablative conditioning regimen to clear out unhealthy blood stem cells and make room for the new ones. This involves a round of high-dose chemotherapy with an agent such as busulfan.
Patients also may receive preventive treatment against seizures prior to myeloablative conditioning.
Casgevy administration
After myeloablative conditioning, a patient’s specific formulation of Casgevy will be administered at a treatment center via a stem cell transplant. This involves a single intravenous infusion of Casgevy at a weight-based dose, with a minimum recommended dose of 3 million cells per kilogram of body weight. Vials of Casgevy are unique to each patient, and the number of vials infused during treatment will vary.
Prior to the infusion, patients also will receive fever-reducing and antihistamine medications to minimize immune reactions.
After receiving Casgevy, patients will need to stay in the treatment center as they recover. The amount of time before going home is around 4-6 weeks, and will be determined by a healthcare provider.
Casgevy in sickle cell disease clinical trials
CRISPR and Vertex’s regulatory applications seeking Casgevy’s approval for SCD were largely backed by data from the Phase 2/3 CLIMB-121 trial (NCT03745287).
CLIMB-121 trial and CLIMB-131 extension study
The open-label CLIMB-121 trial enrolled 63 adults and adolescents, ages 12-35, with severe SCD who were experiencing recurrent VOCs, defined as at least two severe crises per year in the two years before screening. All received a single intravenous infusion of Casgevy after a conditioning regimen with busulfan, and are being followed for two years.
The study’s primary efficacy outcome measure was to assess the proportion of patients who did not experience any severe VOCs for at least a year within the two years after receiving Casgevy.
As of the most recent interim analysis, a total of 58 patients had begun the stem cell mobilization process and 44 had received Casgevy.
Of the 30 patients with evaluable follow-up data, 29 (97%) achieved this outcome, having gone a mean of 22.4 months — just shy of two years — without any severe VOCs. Moreover, all 30 patients with available data avoided hospitalizations due to severe VOCs for at least a year.
Subgroup analyses also indicated the positive effects of Casgevy on VOCs and VOC-related hospitalizations were observed in all patients, regardless of age, sex, or number of pain crises experienced previously.
Biomarker data from the trial also showed Casgevy was able to rapidly and sustainably increase both fetal and total hemoglobin levels, while reducing those of several markers of hemolysis, or red blood cell destruction.
Patient-reported measures of pain and quality of life also tended to improve following treatment with Casgevy.
The stem cell transplant was effective in all patients, with no evidence of transplant rejection observed.
Participants who complete CLIMB-121 have the option of entering in the CLIMB-131 extension study (NCT04208529), in which they will continue to be monitored for up to 15 years. So far, 17 patients have completed the two-year CLIMB-121 trial and enrolled in the long-term extension study.
Other ongoing trials
An ongoing open-label Phase 3 trial called CLIMB-151 (NCT05329649) is now evaluating Casgevy’s safety and efficacy in 15 pediatric patients, ages 2-11, with severe SCD. The trial, which is taking place at sites in the U.S. and Europe, is set to finish in 2026.
A Phase 3b trial (NCT05477563) is evaluating the safety and efficacy of Casgevy in up to 18 people with TDT or severe SCD, ages 12-35, at sites in the U.S., Germany, and Italy. The main goal is to evaluate changes in total and fetal hemoglobin levels over time. That study is expected to finish in 2025.
Another planned Phase 3 trial (NCT05951205) will evaluate Casgevy in up to 12 people with severe SCD, ages 12-35, who have certain types of SCD-causing mutations. Set to begin at the start of 2024, that trial will end in 2029.
Common side effects of Casgevy
The most common side effects reported in clinical trials of Casgevy include:
- inflammation of the mucus membranes lining the mouth and gastrointestinal tract, known as mucositis
- decreased appetite
- low counts of immune neutrophils accompanied by fever, called febrile neutropenia
- low counts of other blood cell types, including platelets, white blood cells, and red blood cells.
Engraftment failure or engraftment delays
After a hematopoietic stem cell (HSC) transplant, cells must engraft, or implant and multiply, within the bone marrow, before they can repopulate the blood with red and white blood cells. A failure or delay in this process can lead to deficiencies in certain blood cell types.
An engraftment failure of neutrophils, a type of immune cells that help fight off infections, is a risk with HSC transplants, but has not been observed in clinical trials of Casgevy. Neutrophil counts should be monitored in all patients. Should a neutrophil engraftment failure occur, a rescue infusion of unmodified stem cells may be required.
Patients who received Casgevy in clinical trials experienced a slower median engraftment time for platelets — small cell fragments involved in blood clotting — compared with traditional HSC transplants. This can lead to an increased risk of bleeding until platelet engraftment is achieved. All individuals should be monitored for bleeding and platelet counts until engraftment is achieved.
Transplant patients should tell their healthcare providers right away if they experience symptoms such as fever, chills, or infections, as this may be a sign of low white blood cell counts. Severe headaches, abnormal bruising, prolonged bleeding, bleeding in the absence of an injury, coughing up blood, or seeing blood in urine, stool, or vomit, also may be signs of low platelet counts.
Allergic reactions
Allergic or hypersensitivity reactions, including life-threatening ones called anaphylaxis, can occur due to certain ingredients used to preserve Casgevy, namely dimethyl sulfoxide or dextran 40. Patients should be monitored for hypersensitivity reactions during and after receiving an infusion of Casgevy.
Off-target DNA editing
There is a possible risk with gene-editing therapies that the technology used may mistakenly edit unintended stretches of DNA other than the target gene. That risk cannot be ruled out with Casgevy. However, this was not observed in Casgevy studies involving cells from healthy donors or patients. The possible clinical significance of off-target gene-editing in terms of safety or efficacy is not known.
Use in pregnancy and breastfeeding
There are no clinical or preclinical data on the use of Casgevy during pregnancy or breastfeeding. However, the myeloablative conditioning regimen that must be completed before the infusion can pose some health risks.
As such, Casgevy should not be administered during pregnancy. Women with reproductive potential must obtain a negative blood pregnancy test prior to each round of stem cell collection, which must be reconfirmed prior to undergoing myeloablative conditioning.
There are insufficient data to inform how long contraception will be necessary following treatment with Casgevy. But both male and female patients with reproductive potential should use an effective contraception method from the time of stem cell collection through to at least six months after receiving Casgevy. Pregnancy thereafter should be discussed with a healthcare provider.
Due to the potential risks of the conditioning regimen, breastfeeding also should be discontinued when patients are undergoing myeloablative conditioning. The health benefits of breastfeeding should be considered, along with the mother’s need for Casgevy and any potential risks to the breastfed child. Breastfeeding after Casgevy should be discussed with a doctor.
It is not known whether Casgevy can lead to fertility problems, but infertility has been observed with myeloablative conditioning. As such, interested patients should discuss fertility preservation options with their healthcare providers before treatment.
Sickle Cell Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Blood test may improve kidney damage detection in children with SCD
- Understanding the impact of leg ulcers in sickle cell disease
- Asthma seven times more likely in SCD children than in unaffected siblings
- Sickle cell drug at high dose eases anemia symptoms in severe SCD
- Researchers urge more talk on menstrual pain in sickle cell clinics
- Sickle cell patients shifting to adult care visit ER more often: US study
- Early results of trial testing tebapivat in SCD expected later this year
- Gathering new evidence helps me tackle my fears with sickle cell
- Differences in red blood cell stiffness may explain variations in SCD severity
- Don’t let sickle cell pain crises keep you from setting goals
Related articles
 Fact-checked by
Fact-checked by